Transforming Cyclopropane To Propene: A Comprehensive Guide
Have you ever wondered about the fascinating transformation of cyclopropane to propene? This chemical conversion is a cornerstone in organic chemistry, offering insights into molecular rearrangements and reaction mechanisms. The process plays a crucial role in industrial applications, academic research, and even pharmaceutical synthesis. Understanding the dynamics of this transformation opens doors to innovation in material science and chemical engineering.
At its core, the conversion of cyclopropane to propene demonstrates the beauty of chemical reactivity. Cyclopropane, a strained, three-membered ring, undergoes a structural rearrangement to form propene, a more stable alkene. This transformation is not just a theoretical concept but a practical tool utilized in various industries. It highlights the delicate interplay of strain energy, bond breaking, and bond formation, offering a deeper appreciation for the intricacies of molecular chemistry.
In this article, we will delve into the step-by-step process of converting cyclopropane to propene. We will explore the underlying principles, reaction conditions, catalytic methods, and industrial implications. Whether you are a student, a researcher, or simply someone intrigued by the wonders of chemistry, this guide will provide you with a thorough understanding of this essential chemical transformation. Let's dive into the science behind cyclopropane to propene!
Read also:Olwen Foueacutereacute A Tale Of Talent And Triumph
Table of Contents
- What Is Cyclopropane?
- What Is Propene?
- Why Is the Conversion Important?
- How Does Cyclopropane to Propene Conversion Happen?
- Reaction Mechanism in Detail
- What Is the Role of Catalysts in This Conversion?
- Industrial Applications of Cyclopropane to Propene
- Safety Considerations in the Conversion Process
- What Is the Environmental Impact of the Conversion?
- Laboratory Demonstration of the Conversion
- Computational Chemistry Perspective
- Future Prospects in Organic Chemistry
- Common Misconceptions About Cyclopropane to Propene
- Frequently Asked Questions
- Conclusion
What Is Cyclopropane?
Cyclopropane is a simple cycloalkane with the molecular formula C3H6. It is characterized by a three-membered ring structure, where the carbon atoms are connected in a triangular arrangement. This unique structure gives cyclopropane significant ring strain due to the deviation from the ideal tetrahedral bond angle of 109.5°.
Here are some key characteristics of cyclopropane:
- Highly strained molecular structure due to bond angle compression.
- Colorless gas at room temperature with a faint, sweet odor.
- Used historically as an anesthetic, though its application has declined due to flammability concerns.
- Chemically reactive, making it a valuable intermediate in organic synthesis.
The high energy associated with cyclopropane's strained bonds makes it susceptible to ring-opening reactions, such as its conversion to propene. This reactivity is a cornerstone of its utility in chemical transformations.
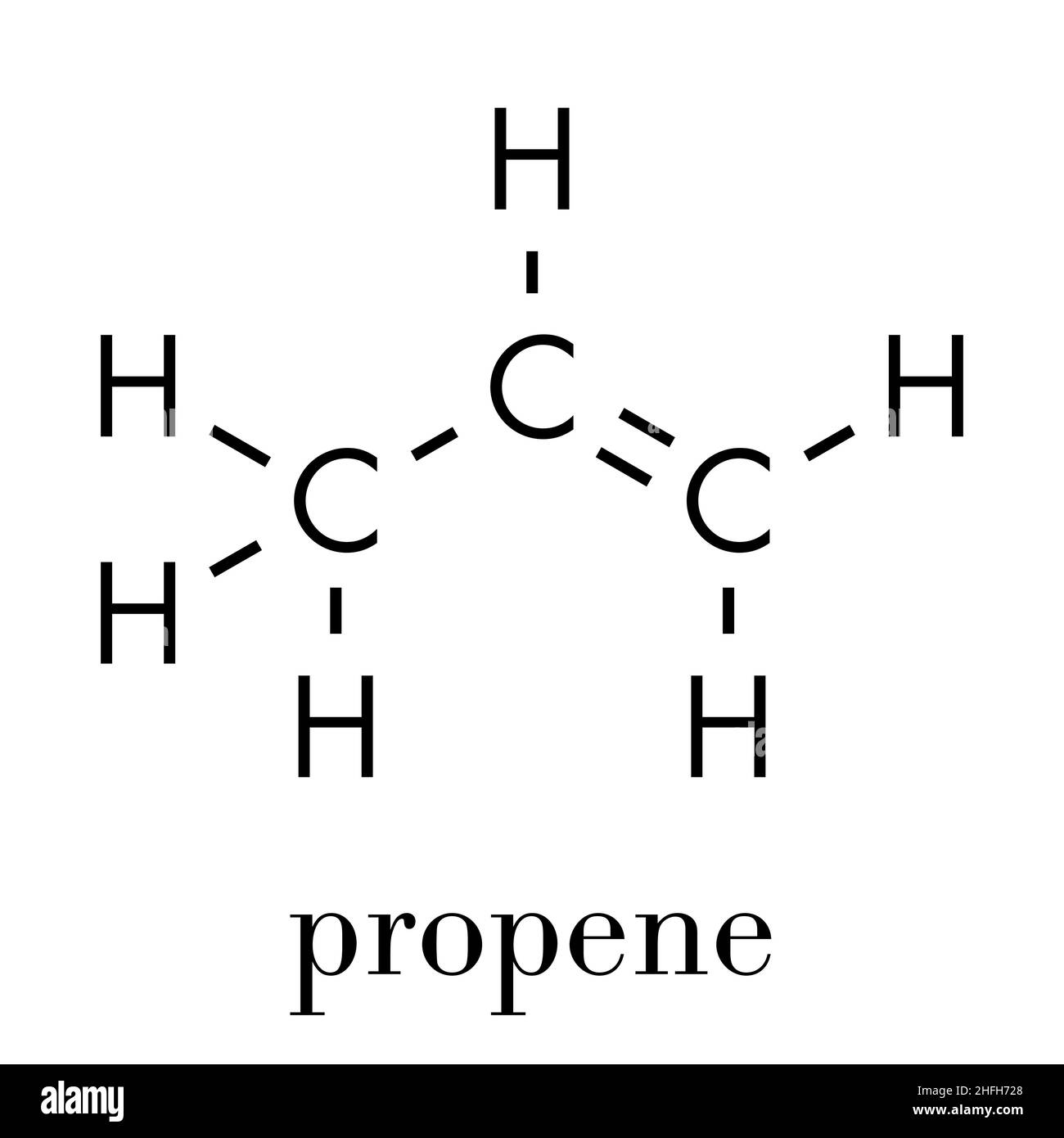
What Is Propene?
Propene, also known as propylene, is an important alkene with the molecular formula C3H6. It contains a double bond between two of its carbon atoms, distinguishing it as an unsaturated hydrocarbon. Propene is a versatile building block in the petrochemical industry, widely used in the production of plastics, chemicals, and fuels.
Key properties of propene include:
- Colorless gas with a faint petroleum-like odor.
- Highly flammable and forms explosive mixtures with air.
- Used in the synthesis of polypropylene, a common plastic material.
- Serves as a precursor for various industrial chemicals, such as propylene oxide and acrylonitrile.
The transition from cyclopropane to propene involves breaking the strained cyclopropane ring and forming a stable double bond, highlighting the importance of structural stability in chemical compounds.
Read also:Pat Summerall The Voice Of American Sports
Why Is the Conversion Important?
The transformation of cyclopropane to propene holds significant importance in both academic and industrial contexts. Here’s why:
- Fundamental Understanding: The conversion serves as a practical example of ring strain and its influence on chemical reactivity.
- Industrial Relevance: Propene is a critical starting material for numerous industrial processes, making its production from cyclopropane economically valuable.
- Educational Value: Demonstrating this conversion in a laboratory setting helps students grasp key concepts in organic chemistry.
- Research Applications: Studying the mechanism provides insights into reaction kinetics, catalyst design, and energy considerations.
Moreover, the cyclopropane to propene conversion exemplifies the broader principle of strain relief in chemistry, where molecules transition from high-energy states to more stable configurations.
How Does Cyclopropane to Propene Conversion Happen?
The transformation of cyclopropane to propene primarily involves a ring-opening reaction, where the three-membered ring of cyclopropane breaks to form a linear structure. This rearrangement is facilitated by the release of ring strain, which drives the reaction toward the formation of the more stable alkene, propene.
Here’s a simplified overview of the process:
- The strained three-membered ring undergoes homolytic or heterolytic bond cleavage.
- The resulting intermediates rearrange to form a double bond.
- The final product, propene, is more stable due to the relief of ring strain and the formation of a conjugated system.
Various methods, such as thermal activation, catalytic hydrogenation, or photochemical processes, can initiate this reaction. The specific conditions depend on the desired efficiency, yield, and application.
Reaction Mechanism in Detail
The detailed mechanism of cyclopropane to propene conversion can be described through a step-by-step analysis of bond-breaking and bond-forming events. This section will explore the thermodynamic and kinetic aspects of the reaction, as well as the role of intermediates and transition states.
Step 1: Initiation
The reaction begins with the cleavage of one of the C-C bonds in the cyclopropane ring. This step may be facilitated by heat, light, or a catalyst, depending on the method employed.
Step 2: Formation of Intermediates
The bond cleavage generates reactive intermediates, such as carbocations, carbanions, or radicals. These species are highly reactive and quickly rearrange to minimize energy.
Step 3: Bond Rearrangement
The intermediates undergo a structural rearrangement, resulting in the formation of a double bond. This step is driven by the thermodynamic stability of the alkene product.
Step 4: Product Formation
The final product, propene, is formed as the reaction reaches completion. The process is typically exothermic, releasing energy as the strained cyclopropane ring is relieved.
Understanding these steps provides valuable insights into the principles of organic reaction mechanisms and the factors influencing chemical reactivity.
What Is the Role of Catalysts in This Conversion?
Catalysts play a pivotal role in the cyclopropane to propene conversion by lowering the activation energy required for the reaction. Common catalysts include transition metals, acids, and bases, each offering unique advantages in terms of reaction rate and selectivity.
For example:
- Transition Metals: Catalysts like platinum, palladium, or nickel can facilitate hydrogenation reactions, promoting the ring-opening process.
- Acids: Strong acids, such as sulfuric acid, can protonate cyclopropane, making it more susceptible to bond cleavage.
- Bases: Basic catalysts can abstract protons, generating reactive intermediates that lead to propene formation.
Choosing the right catalyst depends on factors such as reaction conditions, desired yield, and environmental considerations.
Industrial Applications of Cyclopropane to Propene
The conversion of cyclopropane to propene has several industrial applications, particularly in the petrochemical and polymer industries. Propene serves as a precursor for the production of:
- Polypropylene, a widely used plastic material.
- Propylene oxide, an essential component in polyurethane foams.
- Acrylonitrile, used in the manufacture of synthetic fibers and resins.
- Alcohols and solvents, through further chemical transformations.
The ability to efficiently convert cyclopropane to propene is crucial for meeting the growing demand for these products, making this transformation a valuable tool in industrial chemistry.
Safety Considerations in the Conversion Process
Handling cyclopropane and propene requires strict adherence to safety protocols due to their flammability and potential health hazards. Key safety measures include:
- Using appropriate ventilation to prevent the accumulation of flammable gases.
- Wearing personal protective equipment, such as gloves and goggles.
- Following standard operating procedures for chemical handling and storage.
- Implementing emergency response plans for accidental releases or fires.
By prioritizing safety, researchers and industry professionals can minimize risks and ensure the successful execution of the conversion process.
What Is the Environmental Impact of the Conversion?
The environmental impact of the cyclopropane to propene conversion depends on factors such as energy consumption, byproduct formation, and waste management. While the reaction itself is relatively clean, the use of certain catalysts or solvents may introduce environmental concerns.
Strategies to mitigate these impacts include:
- Developing green catalytic methods that minimize waste and energy use.
- Recycling and reusing reaction byproducts.
- Adopting sustainable practices in industrial processes.
By focusing on environmental sustainability, the chemical industry can balance economic growth with ecological responsibility.
Laboratory Demonstration of the Conversion
Demonstrating the cyclopropane to propene conversion in a laboratory setting offers a hands-on learning experience for students and researchers. The experiment typically involves:
- Preparing a cyclopropane sample and setting up the reaction apparatus.
- Introducing a catalyst or applying heat to initiate the reaction.
- Monitoring the reaction progress using analytical techniques such as gas chromatography or spectroscopy.
- Isolating and characterizing the propene product to confirm the reaction’s success.
This practical demonstration reinforces theoretical concepts and provides valuable insights into chemical reactivity and reaction mechanisms.
Computational Chemistry Perspective
Computational chemistry offers a powerful tool for studying the cyclopropane to propene conversion. By simulating the reaction at the molecular level, researchers can:
- Predict reaction pathways and intermediates.
- Calculate activation energies and thermodynamic properties.
- Optimize reaction conditions for maximum efficiency.
- Design novel catalysts based on theoretical insights.
The integration of computational and experimental approaches enhances our understanding of this transformation and its broader implications in organic chemistry.
Future Prospects in Organic Chemistry
The study of cyclopropane to propene conversion continues to inspire advancements in organic chemistry. Future research directions include:
- Exploring alternative reaction pathways and mechanisms.
- Developing environmentally friendly catalysts and reagents.
- Applying the principles of this conversion to other strained-ring systems.
- Integrating this knowledge into the design of new materials and pharmaceuticals.
By pushing the boundaries of what is possible, chemists can unlock new opportunities for innovation and discovery.
Common Misconceptions About Cyclopropane to Propene
Despite its importance, several misconceptions surround the cyclopropane to propene conversion. Let’s address some of the most common ones:
- Misconception: The reaction is always spontaneous.
Fact: The reaction requires specific conditions, such as heat or catalysts, to proceed efficiently. - Misconception: Cyclopropane is unstable in all conditions.
Fact: Cyclopropane is stable under standard conditions but reacts readily when activated. - Misconception: All catalysts work equally well.
Fact: The choice of catalyst significantly influences reaction rate and selectivity.
By dispelling these myths, we can foster a more accurate understanding of this fascinating transformation.
Frequently Asked Questions
- What is the primary driving force for the cyclopropane to propene conversion?
The release of ring strain in cyclopropane drives the reaction toward the formation of the more stable propene. - Can this conversion occur without a catalyst?
Yes, but the reaction typically requires heat or light to proceed without a catalyst. - What are the industrial applications of propene?
Propene is used in the production of polypropylene, propylene oxide, and various other chemicals and materials. - Are there environmental concerns associated with this reaction?
While the reaction is relatively clean, the use of certain catalysts and solvents may introduce environmental issues. - How can this reaction be demonstrated in a laboratory?
A laboratory demonstration involves preparing cyclopropane, introducing a catalyst, and monitoring the reaction progress using analytical techniques. - What role does computational chemistry play in this transformation?
Computational chemistry helps predict reaction pathways, calculate energy changes, and design efficient catalysts.
Conclusion
The cyclopropane to propene conversion is a cornerstone of organic chemistry, showcasing the interplay of strain energy, reaction mechanisms, and chemical reactivity. From its theoretical principles to its practical applications, this transformation offers valuable insights for students, researchers, and industry professionals alike.
By understanding the science behind this process, we can harness its potential for innovation in fields ranging from materials science to pharmaceutical development. As research continues to push the boundaries of what is possible, the cyclopropane to propene conversion will undoubtedly remain a topic of fascination and importance in the world of chemistry.
Article Recommendations