Polar Vs Nonpolar Covalent Bonds: Key Differences And Real-World Applications
Chemical bonding forms the foundation of modern chemistry, influencing everything from the structure of molecules to the properties of substances. Among the fundamental types of chemical bonds, covalent bonds hold a special place due to their role in forming countless compounds essential for life and technology. But what makes one covalent bond polar and another nonpolar? How do these differences affect the properties of molecules and their real-world applications?
Covalent bonding occurs when atoms share electrons to achieve a stable electron configuration, typically aiming for a full outer shell. However, not all covalent bonds are created equal. The distinction between polar and nonpolar covalent bonds lies in the unequal or equal sharing of electrons, which is influenced by the electronegativity of the atoms involved. These variations can seem subtle but have profound implications on molecular behavior, from solubility and melting points to biological interactions and chemical reactivity.
In this comprehensive guide, we’ll explore the concept of polar vs nonpolar covalent bonds, breaking down their definitions, characteristics, and examples. Through a detailed analysis, we’ll also examine how these bonds influence molecular geometry, chemical properties, and their applications in fields like medicine, environmental science, and materials engineering. By the end of this article, you’ll have a clear understanding of why this seemingly simple distinction is so crucial in chemistry and beyond.
Read also:Comprehensive Guide To Tennis Channel A Musthave For Tennis Enthusiasts
Table of Contents
- What Are Covalent Bonds?
- How Do Covalent Bonds Form?
- What Makes a Bond Polar or Nonpolar?
- Polar Covalent Bonds Explained
- Nonpolar Covalent Bonds Explained
- Differences Between Polar and Nonpolar Covalent Bonds
- Real-Life Examples of Polar Covalent Bonds
- Real-Life Examples of Nonpolar Covalent Bonds
- How Do Polar Bonds Affect Molecular Properties?
- How Do Nonpolar Bonds Affect Molecular Properties?
- Why Are Polar and Nonpolar Bonds Important?
- How Can You Determine If a Bond Is Polar or Nonpolar?
- Common Misconceptions About Polar and Nonpolar Bonds
- FAQs About Polar vs Nonpolar Covalent Bonds
- Conclusion
What Are Covalent Bonds?
Covalent bonds are a type of chemical bond that involves the sharing of electron pairs between atoms. These bonds are fundamental in forming molecules and compounds, ranging from simple diatomic molecules like oxygen (O2) to complex macromolecules like proteins and DNA.
Key characteristics of covalent bonds include:
- Electron Sharing: Atoms share electrons to achieve a stable electronic configuration.
- Strong Bonding: Covalent bonds are generally strong, requiring significant energy to break.
- Formation Between Nonmetals: Typically, covalent bonds form between nonmetallic elements.
Understanding the basics of covalent bonding is essential for differentiating between polar and nonpolar covalent bonds, which are variations of this fundamental bond type.
How Do Covalent Bonds Form?
The formation of covalent bonds is driven by the need for atoms to achieve a more stable, lower-energy state. This usually involves completing their outer electron shells, often referred to as achieving a "full octet."
The steps involved in covalent bond formation include:
- Proximity of Atoms: Two atoms come close enough for their outer electron orbitals to overlap.
- Electron Sharing: The atoms share one or more pairs of electrons.
- Stable Configuration: By sharing electrons, each atom attains a stable electronic structure.
For example, in the hydrogen molecule (H2), two hydrogen atoms share one pair of electrons, resulting in a single covalent bond.
Read also:Celebrating Aaliyah The Timeless Icon Of Rampb
What Makes a Bond Polar or Nonpolar?
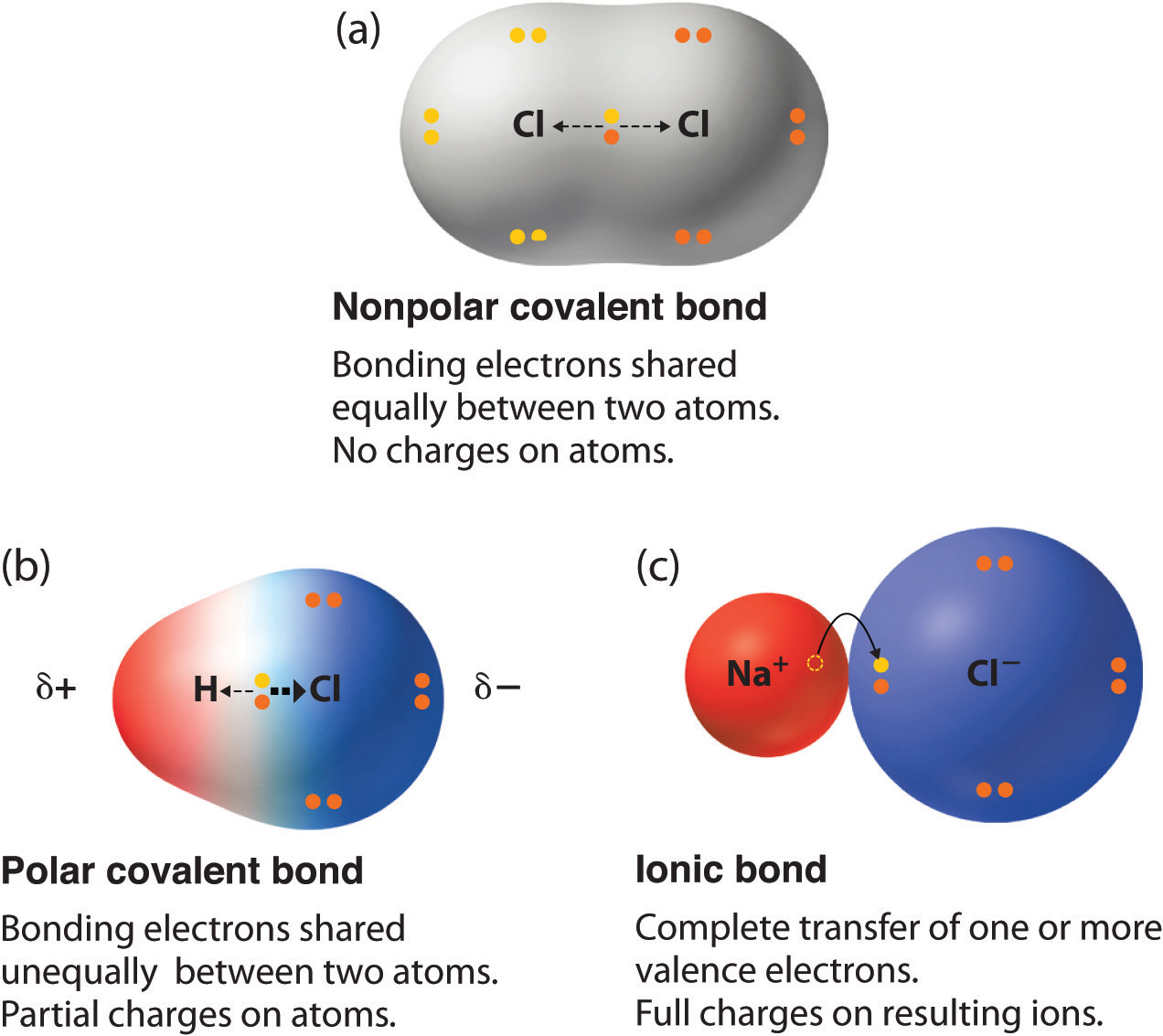
The distinction between polar and nonpolar covalent bonds lies in the distribution of electron density between the bonded atoms. This is influenced by the concept of electronegativity, which refers to an atom's ability to attract shared electrons.
Here’s how it works:
- Equal Sharing: In nonpolar covalent bonds, the atoms have similar electronegativities, leading to an equal sharing of electrons.
- Unequal Sharing: In polar covalent bonds, one atom has a higher electronegativity, causing electrons to be shared unequally.
The result is a partial charge separation in polar bonds, whereas nonpolar bonds show no such charge asymmetry.
Polar Covalent Bonds Explained
Polar covalent bonds occur when two atoms share electrons unequally due to a difference in their electronegativities. This results in a molecule with a partial positive charge on one side and a partial negative charge on the other.
Examples include:
- Water (H2O): The oxygen atom is more electronegative than hydrogen, causing the shared electrons to spend more time near the oxygen nucleus.
- Hydrogen Chloride (HCl): Chlorine, being more electronegative, pulls the shared electron pair closer to itself.
Polar covalent bonds play a critical role in many chemical and biological processes, such as hydrogen bonding and solubility in water.
Nonpolar Covalent Bonds Explained
Nonpolar covalent bonds occur when two atoms share electrons equally, typically because they have identical or very similar electronegativities. This results in a balanced distribution of electron density.
Examples include:
- Molecular Oxygen (O2): Both oxygen atoms have the same electronegativity, leading to equal sharing of electrons.
- Methane (CH4): Although carbon and hydrogen have slightly different electronegativities, the geometry of the molecule makes it nonpolar overall.
Nonpolar bonds are essential in forming molecules like fats and oils, which are hydrophobic and do not mix with water.
Differences Between Polar and Nonpolar Covalent Bonds
Understanding the differences between polar and nonpolar covalent bonds is essential for predicting molecular behavior and interactions. The table below summarizes the key distinctions:
| Aspect | Polar Covalent Bonds | Nonpolar Covalent Bonds |
|---|---|---|
| Electron Sharing | Unequal | Equal |
| Electronegativity Difference | Significant | Minimal or None |
| Charge Distribution | Partial Charges | Neutral |
| Examples | H2O, HCl | O2, CH4 |
Real-Life Examples of Polar Covalent Bonds
Polar covalent bonds are ubiquitous in nature and play a critical role in various applications. Some real-life examples include:
- Water (H2O): The polar nature of water makes it an excellent solvent for ionic and polar substances.
- Ammonia (NH3): Its ability to form hydrogen bonds makes it useful in refrigeration systems.
- Ethanol (C2H5OH): The polar OH group enables its use as a solvent in various industries.
These molecules demonstrate how polar covalent bonds influence solubility, boiling points, and intermolecular interactions.
Real-Life Examples of Nonpolar Covalent Bonds
Nonpolar covalent bonds are equally significant and find applications in many areas. Examples include:
- Molecular Nitrogen (N2): The inert nature of N2 is vital for creating a protective atmosphere in industrial processes.
- Hexane (C6H14): Its nonpolarity makes it an ideal solvent for nonpolar substances.
- Carbon Dioxide (CO2): Despite having polar bonds, the linear geometry of CO2 makes it nonpolar overall.
These examples highlight the role of nonpolar covalent bonds in creating molecules with unique properties.
FAQs About Polar vs Nonpolar Covalent Bonds
- Can a molecule have both polar and nonpolar bonds?
Yes, molecules like ethanol can have both polar (OH group) and nonpolar (CH chain) bonds. - How does molecular geometry affect polarity?
Geometry determines the overall dipole moment, making some molecules with polar bonds nonpolar overall. - Are ionic bonds stronger than polar covalent bonds?
Generally, ionic bonds are stronger due to the complete transfer of electrons. - Why is water considered a universal solvent?
Its polar nature allows it to dissolve a wide range of substances. - What role do polar bonds play in biological systems?
Polar bonds are crucial for hydrogen bonding, enzyme activity, and molecular recognition. - Can nonpolar bonds conduct electricity?
No, nonpolar bonds lack free electrons or ions required for electrical conductivity.
Conclusion
The distinction between polar and nonpolar covalent bonds is a cornerstone of chemistry, influencing molecular behavior, properties, and applications. By understanding their differences and implications, scientists and engineers can design materials, develop medicines, and solve real-world challenges. Whether you're studying chemistry or exploring its practical applications, grasping the concept of polar vs nonpolar covalent bonds is essential for unlocking the potential of molecular science.
Article Recommendations